Consider the reaction coordinate diagram shown. Which step has the greatest activation energy? 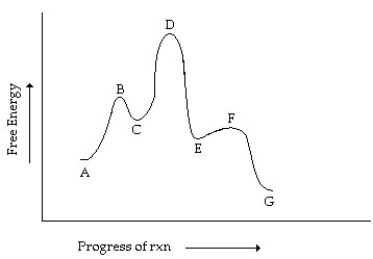
A) A going to C
B) C going to E
C) E going to G
D) E going to C
E) C going to A
Correct Answer:
Verified
Q60: Assign the E or Z configurational label
Q61: Consider the reaction coordinate diagram shown. Which
Q62: Which of the following correctly describes the
Q63: Which of the following correctly describes the
Q63: Under what conditions is ΔG° equal to
Q67: Based on the following energy diagram, which
Q68: Why do reactions tend to proceed at
Q68: Consider the reaction coordinate diagram shown. Which
Q69: Consider the single step interconversion of A
Q78: Consider the conversion of C to D
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents