Why is the alkyl halide below not capable of undergoing an E2 reaction upon treatment with sodium ethoxide? 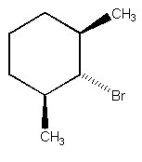
A) Br- is too poor a leaving group.
B) The substrate is too hindered.
C) Too much angle strain would be present in the alkene product.
D) Sodium ethoxide is a poor base to use in E2 reactions.
E) The C-H and C-Br bonds which need to break cannot achieve an anti-periplanar orientation.
Correct Answer:
Verified
Q44: Provide the structure of the major organic
Q45: Give the major product for the following
Q50: Provide the structure of the major organic
Q52: Identify the alkyl halide that reacts the
Q53: A primary kinetic isotope effect could most
Q54: Give the major product for the following
Q107: Which diastereomer of 1-bromo-4-t-butylcyclohexane, the cis or
Q165: Which of the following is least likely
Q171: What is the major product which results
Q178: Why is the E1 not a likely
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents