Which of the following is the rate-determining step for the monobromination of cyclohexane? 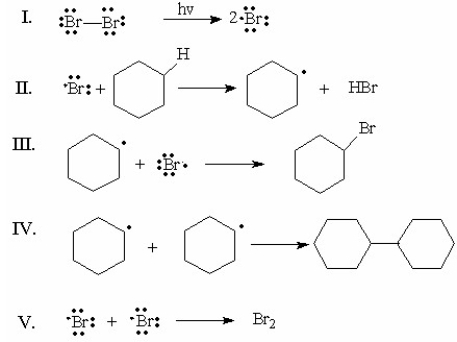
A) I
B) II
C) III
D) IV
E) V
Correct Answer:
Verified
Q1: During the free radical chlorination of methane,which
Q3: Given the bond dissociation energies below (in
Q4: How many dichlorinated products, including stereoisomers, can
Q5: How many products are formed from the
Q5: Which of the following is a chain
Q7: The reaction Br2 + CH3Br → CH2Br2
Q10: The major type of reactions that alkanes
Q10: Which of the following products result from
Q13: The reaction Br2 + CH3Br → CH2Br2
Q17: Explain why alkanes are generally considered unreactive
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents