What is the main purpose for using metal-ions as catalysts in the following hydrolysis reaction? 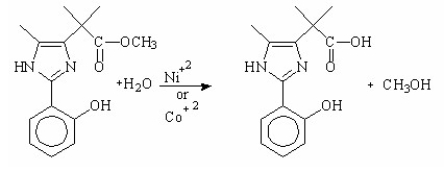
A) increases the rate by making water a stronger base, thereby increasing its electrophilicity
B) increases the rate by making water a stronger base, thereby increasing its nucleophilicity
C) increases the rate by making water a stronger acid, thereby increasing its nucleophilicity
D) increases the rate by making water a stronger acid, thereby increasing its electrophilicity
E) increases the rate by making water a stronger acid, thereby decreasing its nucleophilicity
Correct Answer:
Verified
Q21: How does Zn2+ catalyze the hydrolysis of
Q27: Hydrolysis of esters occurs more rapidly in
Q29: Which of the following is not true
Q30: Offer an explanation of how iodide catalyzes
Q32: How does a metal catalyst increase the
Q34: Which of the following is an intermediate
Q34: How does Cu2+ catalyze the decarboxylation of
Q42: Which of the following is not true
Q58: Explain the phenomenon known as molecular recognition.Give
Q60: Which of the following is true of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents