Reaction of A (unshaded spheres) with B2 (shaded spheres) is shown schematically in the following diagram.Which equation best describes the stoichiometry of the reaction? 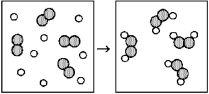
A) A2 + 2 B → A2B2
B) 8 A + 4 B2 → 4 A2B2
C) 2 A + B2 → A2B2
D) 4 A + 4 B2 → 4 A2B2
Correct Answer:
Verified
Q71: Q72: What is the balanced chemical equation for Q73: Q74: The following diagram represents the reaction of Q75: Aluminum metal reacts with aqueous copper(II)sulfate to Q77: If unshaded spheres represent nitrogen atoms and Q78: The following diagrams represent the reaction of Q79: If unshaded spheres represent nitrogen atoms and Q80: What is the balanced chemical equation for Q81: What mass of ammonia,N H3,contains the same![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents