Multiple Choice
For the fourth-shell orbital shown below,what are the principal quantum number,n,and the angular momentum quantum number,l? 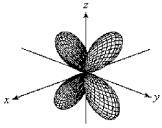
A) n = 4 and l = 0
B) n = 4 and l = 1
C) n = 4 and l = 2
D) n = 4 and l = 3
Correct Answer:
Verified
Related Questions
Q56: Which of the following is true? The
Q57: If the quantum number ms had possible
Q58: Which statement is false?
A)For any atom,the 4s
Q59: The first vibrational level for NaH lies
Q60: The symbol [Kr] represents
A)4s24p6.
B)1s22s22p63s23p64s24p6.
C)1s22s22p63s23p63d104s24p6.
D)1s22s22p63s23p63d104s24p64d10.

