Multiple Choice
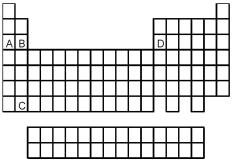
-Atoms of which element,indicated by letter on the periodic table,would be expected to have the most negative value of Eea? 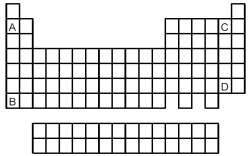
A) A
B) B
C) C
D) D
Correct Answer:
Verified
Related Questions
Q60: Calculate the energy change for the formation
Q61: Q62: The four spheres below represent K+,Ca2+,Cl-,and S2-,not Q63: Q64: The four spheres below represent Na+,Mg2+,F⁻,and O2-,not Q66: Q67: Q68: The four spheres below represent K+,Ca2+,Cl-,and S2-,not Q69: The four spheres below represent K+,Ca2+,Cl-,and S2-,not Q70: Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()
![]()
![]()

