Which substance in each of the following pairs is expected to have the larger dispersion forces? 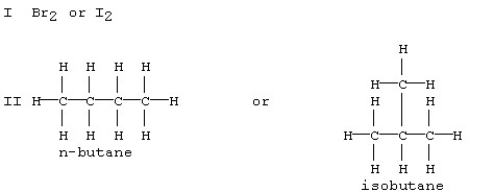
A) Br2 in set I and n-butane in set II
B) Br2 in set I and isobutane in set II
C) I2 in set I and n-butane in set II
D) I2 in set I and isobutane in set II
Correct Answer:
Verified
Q16: Which of the following is not a
Q17: What is the molecular geometry of CH3-?
A)T-shaped
B)tetrahedral
C)trigonal
Q18: Which type of bond produces a charge
Q19: What is the molecular geometry of TeF5-?
A)octahedral
B)seesaw
C)square
Q20: Which orbital hybridization is associated with a
Q22: The MO diagram below is appropriate for
Q23: Which of the following molecules will readily
Q24: The HI bond has a length of
Q25: Which compound below could have a zero
Q26: Which molecular orbital resembles a d-orbital?
A)σ
B)σ*
C)π
D)π*
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents