Which molecule has a central atom that uses the set of hybrid orbitals shown below to form bonds with the non-central atoms? 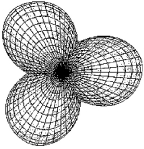
A) H2S
B) CS2
C) NO2-
D) IF2-
Correct Answer:
Verified
Q187: The molecular geometry of OCCl2 is _.
Q188: Of XeF2 and XeF4,the one with the
Q189: Which molecule has a central atom that
Q190: The carbon-carbon bond in C2H2 contains _
Q191: The Br-Cl bond has 5.05% ionic character
Q193: The hybrid orbital used by nitrogen to
Q194: Shown below is a model of PCl5
Q195: The bonds in the polyatomic ion NO3-
Q196: Shown below is a model of SiH4
Q197: Pt(NH3)2Cl2 is square planar.The isomer of Pt(NH3)2Cl2
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents