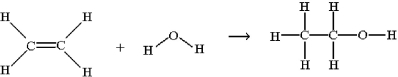
One method for making ethanol,C2H5OH,involves the gas-phase hydration of ethylene,C2H4: Estimate ΔH for this reaction from the given average bond dissociation energies,D. 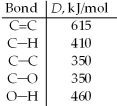
A) -580 kJ
B) -35 kJ
C) +35 kJ
D) 580 kJ
Correct Answer:
Verified
Q52: Find ΔH° for the reaction C3H8(g)+ 5
Q53: Which of CH4(g),C2H2(g),and CH3OH(l)provides the most energy
Q54: Which thermodynamic function is most related to
Q55: For the reaction,C2H2(g)→ 2 C(g)+ 2 H(g),one
Q56: Given: S (s)+ O2 (g)→ SO2 (g)ΔH°
Q58: Which equation represents the reaction whose ΔH,represents
Q59: Use the given standard enthalpies of formation
Q60: Calculate the enthalpy of combustion per mole
Q61: Reactant R reacts with reactant S in
Q62: Consider the conversion of white tin to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents