Calculate the enthalpy of combustion per mole for C6H12O6.Assume that the combustion products are CO2(g) and H2O(l) . 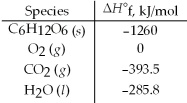
A) -5336 kJ/mol
B) -2816 kJ/mol
C) -1939 kJ/mol
D) 580.7 kJ/mol
Correct Answer:
Verified
Q55: For the reaction,C2H2(g)→ 2 C(g)+ 2 H(g),one
Q56: Given: S (s)+ O2 (g)→ SO2 (g)ΔH°
Q57: One method for making ethanol,C2H5OH,involves the gas-phase
Q58: Which equation represents the reaction whose ΔH,represents
Q59: Use the given standard enthalpies of formation
Q61: Reactant R reacts with reactant S in
Q62: Consider the conversion of white tin to
Q63: For the of freezing liquid butane at
Q64: Which of the following is not true?
A)A
Q65: The enthalpy of fusion of naphthalene,C10H8,is 19.1
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents