Imagine a reaction that results in a change in both volume and temperature,as shown in the diagram below.What is the sign of the work being done and the sign of the enthalpy change involved in this reaction? 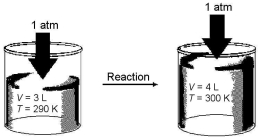
A) w = + and ΔH = +
B) w = + and ΔH = -
C) w = - and ΔH = +
D) w = - and ΔH = -
Correct Answer:
Verified
Q62: Consider the conversion of white tin to
Q63: For the of freezing liquid butane at
Q64: Which of the following is not true?
A)A
Q65: The enthalpy of fusion of naphthalene,C10H8,is 19.1
Q66: For the conversion of water to ice
Q68: Methanol can be produced from carbon monoxide
Q69: If an endothermic reaction is spontaneous at
Q70: The reaction 4 Ag(s)+ O2(g)→ 2 Ag2O(s)favors
Q71: For the expansion of an ideal gas
Q72: Imagine a reaction that results in a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents