Multiple Choice
What are the signs of ΔH,ΔS,and ΔG for the following spontaneous change? 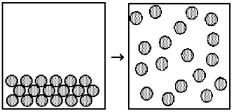
A) ΔH = +,ΔS = +,ΔG = -
B) ΔH = +,ΔS = -,ΔG = -
C) ΔH = -,ΔS = +,ΔG = -
D) ΔH = -,ΔS = -,ΔG = -
Correct Answer:
Verified
Related Questions
Q87: The following drawing is a representation of
Q88: The intermediate,T,+ S is represented by
A)arrow C.
B)line
Q89: For a process at constant pressure,24,800 calories
Q90: At 298 K the average kinetic energy
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents