The following drawing is a representation of the exothermic reaction in which ozone forms dioxygen. 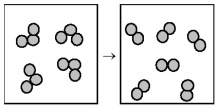
-This reaction is likely to be
A) nonspontaneous at all temperatures.
B) nonspontaneous at low temperatures and spontaneous at high temperatures.
C) spontaneous at low temperatures and nonspontaneous at high temperatures.
D) spontaneous at all temperatures.
Correct Answer:
Verified
Q90: At 298 K the average kinetic energy
Q91: Q91: Q92: What are the signs of ΔH,ΔS,and ΔG Q93: The following drawing is a representation of Q96: The product,Z,is represented by Q97: The following drawing is a representation of Q98: What are the signs of ΔH,ΔS,and ΔG Q99: The net reaction is represented by Q100: Reactant R reacts with reactant S in![]()
![]()
A)arrow A.
B)line D.
C)line E.
D)line
A)arrow A.
B)arrow
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents