The following drawing is a representation of a reaction of the type A → B,where different shaded spheres represent different molecular structures.For this reaction ΔH° = +45 kJ.This reaction is likely to be 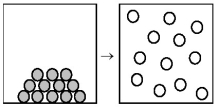
A) nonspontaneous at all temperatures.
B) nonspontaneous at low temperatures and spontaneous at high temperatures.
C) spontaneous at low temperatures and nonspontaneous at high temperatures.
D) spontaneous at all temperatures.
Correct Answer:
Verified
Q82: Q83: The following drawing is a representation of Q84: What are the signs of ΔH,ΔS,and ΔG Q85: The reactants,R + 2 S,are represented by Q86: The following drawing is a representation of Q88: The intermediate,T,+ S is represented by Q89: For a process at constant pressure,24,800 calories Q90: At 298 K the average kinetic energy![]()
A)arrow
A)arrow C.
B)line
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents