Calculate the total quantity of heat required to convert 25.0 g of liquid CCl4(l) from 45.0°C to gaseous CCl4 76.8°C (the normal boiling point for CCl4) ? The specific heat of CCl4(l) is 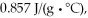 its heat of fusion is
its heat of fusion is 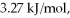 and its heat of vaporization is
and its heat of vaporization is 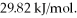
A) 0.681 kJ
B) 1.21 kJ
C) 5.53 kJ
D) 6.06 kJ
Correct Answer:
Verified
Q104: Which of the following is not a
Q105: When 2.00 mol of benzene is vaporized
Q106: At constant pressure for which of the
Q107: Calculate the work,w,gained or lost by the
Q108: How much heat is absorbed/released when 20.00
Q110: How much heat is absorbed when 30.00
Q111: A slice of cheese pizza has a
Q112: At constant pressure,the combustion of 25.0 g
Q113: The first law of thermodynamics
A)defines water energy.
B)defines
Q114: When 6.000 moles of H2(g)reacts with 3.000
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents