You are given two flasks of equal volume.One contains H2 at 0°C and 1 atm while the other contains CO2 at 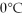 and 2 atm.Which of the following quantities will be the same for both flasks?
and 2 atm.Which of the following quantities will be the same for both flasks?
A) average molecular kinetic energy
B) average molecular speed
C) density
D) number of molecules present
Correct Answer:
Verified
Q69: One reaction that contributes to photochemical smog
Q70: Q71: Which of the following regions of the Q72: The lowest atmospheric temperatures are found at Q73: Which of the following regions of the Q75: An unknown gas effuses 1.73 times faster Q76: At what temperature will sulfur hexafluoride molecules![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents