The plots below represent vapor pressure vs.temperature curves for diethyl ether,ethanol,mercury,and water,not necessarily in that order. 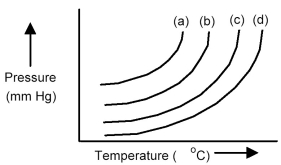
-Based on the relative strengths of the intermolecular forces of attraction of each substance,which is the most likely vapor pressure vs.temperature curve for mercury?
A) curve (a)
B) curve (b)
C) curve (c)
D) curve (d)
Correct Answer:
Verified
Q44: An ionic compound crystallizes in a unit
Q45: A binary ionic compound,MxAy,crystallizes in a cubic
Q46: O2 and O3 are _ of oxygen.
A)allotropes
B)isomers
C)isotopes
D)stereomers
Q47: The edge length of a face-centered cubic
Q48: An ionic compound crystallizes in a unit
Q50: A supercritical fluid refers to a substance
A)above
Q51: The plots below represent vapor pressure vs.temperature
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents