Arrows in the energy diagram below represent enthalpy changes occurring in the exothermic formation of a solution:
ΔHsoln = enthalpy of solution
ΔHsolute-solute = enthalpy change involving solute-solute interactions
ΔHsolute-solvent = enthalpy change involving solute-solvent interactions
ΔHsolvent-solvent = enthalpy change involving solvent-solvent interactions 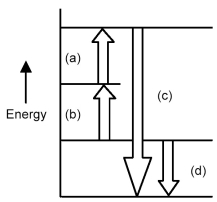
-Which arrow represents ΔHsoln?
A) arrow (a)
B) arrow (b)
C) arrow (c)
D) arrow (d)
Correct Answer:
Verified
Q82: The following diagram shows a close-up view
Q83: Q84: The following diagram shows a close-up view Q85: Drawings (1)and (2)show the equilibrium vapor pressures Q88: The following diagram shows a close-up view Q89: Drawing (1)shows a system in which an![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents