Drawing (1) shows a system in which an equilibrium exists between dissolved and undissolved gas particles at P = 1 atm.According to Henry's law,if the pressure is decreased to 0.5 atm and equilibrium is restored,which drawing (2) -(5) best represents the equilibrium at 0.5 atm? 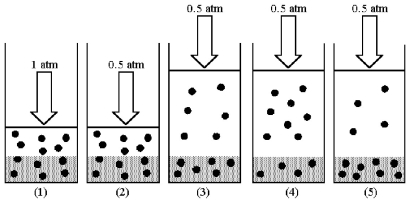
A) drawing (2)
B) drawing (3)
C) drawing (4)
D) drawing (5)
Correct Answer:
Verified
Q87: Arrows in the energy diagram below represent
Q90: Q91: Which ion-dipole interaction results in the larger Q92: A solution of a nonelectrolyte solution contains Q93: Which ion-dipole interaction results in the larger Q94: Arrows in the energy diagram below represent Q97: Arrows in the energy diagram below represent Q97: Arrows in the energy diagram below represent Q98: Q100: Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()

