Substances with high lattice energies tend to be less soluble than substances with low lattice energies.On that basis predict the relative aqueous solubility at 20°C,from highest to lowest,of the following ionic compounds: 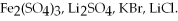
A) Fe2(SO4) 3 > Li2SO4 > KBr > LiCl
B) Fe2(SO4) 3 > Li2SO4 > LiCl > KBr
C) KBr > LiCl > Li2SO4 > Fe2(SO4) 3
D) LiCl > KBr > Li2SO4 > Fe2(SO4) 3
Correct Answer:
Verified
Q117: Which cation in each set would be
Q118: Drawing (1)shows a nonequilibrium system comprised of
Q119: A phase diagram of temperature versus composition
Q120: Which is not a solution?
A)sterling silver
B)fog
C)hydrochloric acid
D)coffee
Q121: To make a 0.125 m solution,one could
Q123: To make a 0.125 M solution,one could
Q124: A solution of LiCl in water is
Q125: What is the mole fraction of ethanol
Q126: What volume of a 0.716 M KBr
Q127: A solution of LiCl in water has
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents