Methanol can be produced by the following reaction: CO(g) + 2 H2(g) → CH3OH(g) .
How is the rate of disappearance of hydrogen gas related to the rate of appearance of methanol?
- 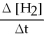 = ?
= ?
A) + 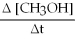
B) + 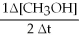
C) +2 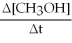
D) none of these
Correct Answer:
Verified
Q3: What is the overall reaction order for
Q4: Chlorine reacts with chloroform according to the
Q5: The following set of data was obtained
Q6: A concentration-time study of the gas phase
Q7: A concentration-time study of the gas phase
Q9: Iodide and hypochlorite ion react in aqueous
Q10: Hydrogen peroxide decomposes to water and oxygen
Q11: Using the method of initial rates for
Q12: The decomposition of dinitrogen pentoxide is described
Q13: For a reaction that follows the general
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents