Consider the first-order reaction A → B in which A molecules (unshaded spheres) are converted to B molecules (shaded spheres) .Given the following pictures at t = 0 seconds and t = 100 seconds,which picture represents the number of A and B molecules remaining at 200 seconds? 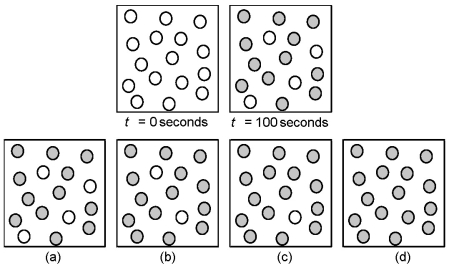
A) picture a)
B) picture b)
C) picture c)
D) picture d)
Correct Answer:
Verified
Q83: The relative initial rates of the reaction
Q84: The following reaction is first order in
Q85: Shown is a concentration versus time plot
Q86: The following reaction is second order in
Q87: Consider the first-order reaction A → B
Q89: The following reaction is first order in
Q90: The relative initial rates of the reaction
Q91: Consider the first-order decomposition of A molecules
Q92: The following reaction is second order in
Q93: Consider the first-order decomposition of A molecules
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents