Write the equilibrium equation for the reverse reaction: 2 CH4(g) + 3 O2(g) ⇌ 2 CO(g) + 4 H2O(g)
A) Kp' = 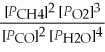
B) Kp' = 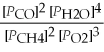
C) Kp' = 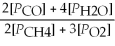
D) Kp' = 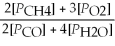
Correct Answer:
Verified
Q1: If Kc is the equilibrium constant for
Q3: Which of the following statements is false
Q4: A mixture of carbon monoxide,hydrogen,and methanol is
Q5: What is true about the relationship of
Q6: Which statement about the equilibrium constant is
Q7: If Kc is the equilibrium constant for
Q8: The oxidation of sulfur dioxide by oxygen
Q9: Which one of the following statements does
Q10: Kp = 1.5 × 103 at 400°C
Q11: The equilibrium equation is also known as
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents