Picture (1) represents an equilibrium mixture of solid CaCO3,solid CaO,and gaseous CO2,obtained as a result of the endothermic decomposition of CaCO3. 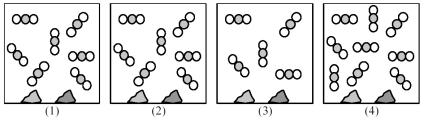
-Which picture (2) -(4) represents the equilibrium mixture when a catalyst is added?
A) picture (2)
B) picture (3)
C) picture (4)
D) All of these
Correct Answer:
Verified
Q104: The equilibrium constant,Kp,equals 3.40 at 25°C for
Q105: The decomposition of ammonia is: 2 NH3(g)⇌
Q106: The equilibrium constant is equal to 5.00
Q107: At some temperature,a 4.0 L flask is
Q108: For which one of the following reactions
Q110: Write the equilibrium equation for the reverse
Q111: Which equilibrium below is homogeneous?
A)CaSO4(s)⇌ Ca2+(aq)+ SO42-(aq)
B)2
Q112: Picture (1)represents an equilibrium mixture of solid
Q113: Write the equilibrium equation for the forward
Q114: Picture (1)represents an equilibrium mixture of solid
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents