Picture (1) represents an equilibrium mixture of solid CaCO3,solid CaO,and gaseous CO2,obtained as a result of the endothermic decomposition of CaCO3. 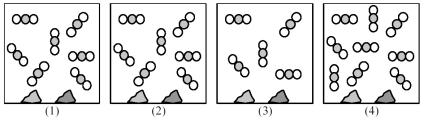
-Which picture (2) -(4) represents the equilibrium mixture when more solid CaCO3 is added?
A) picture (2)
B) picture (3)
C) picture (4)
D) All of these
Correct Answer:
Verified
Q98: The following pictures represent mixtures of A2B4
Q99: The following picture represents the equilibrium state
Q100: Shown below is a concentration vs.time plot
Q101: Picture (1)represents an equilibrium mixture of solid
Q102: Nitric oxide reacts with oxygen to form
Q104: The equilibrium constant,Kp,equals 3.40 at 25°C for
Q105: The decomposition of ammonia is: 2 NH3(g)⇌
Q106: The equilibrium constant is equal to 5.00
Q107: At some temperature,a 4.0 L flask is
Q108: For which one of the following reactions
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents