Iron oxide ores are reduced to iron metal by exothermic reaction with carbon monoxide: 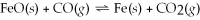 Which of the following changes in condition will cause the equilibrium to shift to the right?
Which of the following changes in condition will cause the equilibrium to shift to the right?
A) remove FeO
B) add CO
C) add CO2
D) raise the temperature
Correct Answer:
Verified
Q126: Phosphorus pentachloride decomposes to phosphorus trichloride at
Q127: "If a stress is applied to a
Q128: For the reaction,A(g)+ 2 B(g)⇌ 2 C(g),Kc
Q129: At a certain temperature,Kc equals 1.4 ×
Q130: Cyclohexane,C6H12,undergoes a molecular rearrangement in the presence
Q132: For the reaction: 4 HCl(g)+ O2(g)⇌ 2
Q133: For the reaction shown below,which change in
Q134: The enthalpy for the following reaction is
Q135: At a certain temperature,hydrogen and iodine react
Q136: Ammonium bromide is a crystalline solid that
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents