The following pictures represent equal volumes of aqueous solutions of three acids HA (A = X,Y,or Z) ;water molecules have been omitted for clarity. 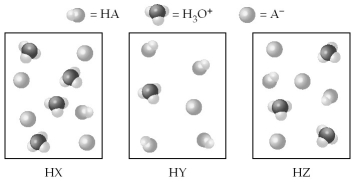
-Arrange the acids in order of increasing value of Ka.
A) Ka(HZ) < Ka(HY) < Ka(HX)
B) Ka(HY) < Ka(HZ) < Ka(HX)
C) Ka(HZ) < Ka(HX) < Ka(HY)
D) Ka(HX) < Ka(HZ) < Ka(HY)
Correct Answer:
Verified
Q99: Dihydrogen phosphate H2PO4-,has an acid dissociation constant
Q100: Calculate the pH of a of 0.100
Q101: Which of the following species cannot act
Q102: The following pictures represent aqueous solutions of
Q103: In the following reaction the unshaded spheres
Q103: In the following reaction the unshaded spheres
Q105: The following pictures represent aqueous solutions of
Q106: For Cu2+ and CO2,which will behave as
Q107: The following pictures represent aqueous solutions of
Q108: The following pictures represent aqueous solutions of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents