The following pictures represent equal volumes of aqueous solutions of three acids HA (A = X,Y,or Z) ;water molecules have been omitted for clarity. 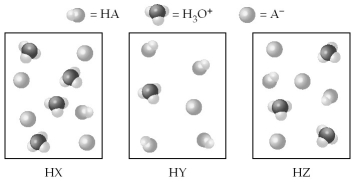
-Arrange the conjugate bases (A- = X-,Y-,or Z-) in order of increasing value of Kb.
A) Kb(Z-) < Kb(Y-) < Kb(X-)
B) Kb(Y-) < Kb(Z-) < Kb(X-)
C) Kb(Z-) < Kb(X-) < Kb(Y-)
D) Kb(X-) < Kb(Z-) < Kb(Y-)
Correct Answer:
Verified
Q134: BF3 and NH3 undergo a Lewis acid-base
Q135: SO3 reacts with H2O to form H2SO4.Which
Q136: The following pictures represent equal volumes of
Q137: Q138: The following pictures represent solutions of three Q140: The following pictures represent solutions of three Q141: In the following chemical equation indicate the Q142: What is the geometric shape of the Q143: A solution with a hydroxide ion concentration Q144: What is the strongest acid among the![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents