SO3 reacts with H2O to form H2SO4.Which picture below correctly represents the curved arrow notation for the initial Lewis acid-Lewis base interaction in this reaction;what is the Lewis acid and the Lewis base? 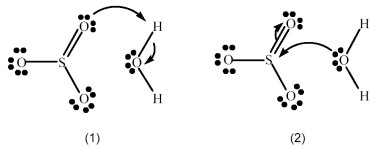
A) Picture (1) is correct;H2O is the Lewis acid and SO3 is the Lewis base.
B) Picture (1) is correct;SO3 is the Lewis acid and H2O is the Lewis base.
C) Picture (2) is correct;H2O is the Lewis acid and SO3 is the Lewis base.
D) Picture (2) is correct;SO3 is the Lewis acid and H2O is the Lewis base.
Correct Answer:
Verified
Q130: An Arrhenius base is best defined as
Q131: Which Br∅nsted-Lowry acid is not considered to
Q132: An Arrhenius acid is best defined as
Q133: The following pictures represent solutions of three
Q134: BF3 and NH3 undergo a Lewis acid-base
Q136: The following pictures represent equal volumes of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents