The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 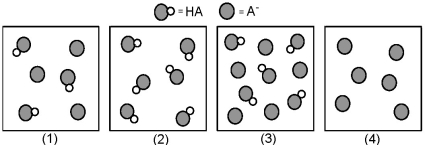
-Which solution has the lowest pH?
A) (1)
B) (2)
C) (3)
D) (4)
Correct Answer:
Verified
Q57: Which of the following metal hydroxides are
Q58: What is the pH of the resulting
Q59: What is the molar solubility of lead(II)chromate
Q60: The dissociation equilibrium constants for the protonated
Q63: The following pictures represent solutions that contain
Q64: A solution may contain the following ions
Q65: 0.10 M potassium chromate is slowly added
Q66: Which metal sulfides can be precipitated from
Q67: The following pictures represent solutions that contain
Q79: The following pictures represent solutions that contain
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents