The following pictures represent solutions of AgCl,which may also contain ions other than Ag+ and Cl- which are not shown.Gray spheres represent Ag+ ions and dotted spheres represent Cl- ions. 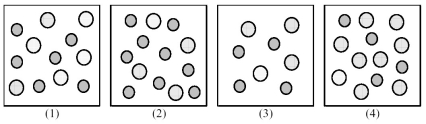
-If solution (1) is a saturated solution of AgCl,which of solutions (1) -(4) represents the solution after a small amount of AgNO3 is added and equilibrium is restored?
A) (1)
B) (2)
C) (3)
D) (4)
Correct Answer:
Verified
Q109: The following pictures represent solutions of CaCO3,which
Q110: The following plot shows a titration curve
Q111: The following pictures represent solutions of CuS,which
Q112: The following pictures represent solutions of AgCl,which
Q113: The following pictures represent solutions of CaCO3,which
Q115: What is the pH at the first
Q116: The following plot shows a titration curve
Q117: The following plot shows a titration curve
Q118: The following pictures represent solutions of CuS,which
Q119: The following pictures represent solutions of CuS,which
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents