What is the silver ion concentration for a saturated solution of SrF2 if the Ksp for SrF2 is 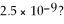
A) 9.3 × 10-7 M
B) 1.4 × 10-3 M
C) 8.5 × 10-4 M
D) 4.06 × 10-4 M
Correct Answer:
Verified
Q156: Sodium hypochlorite,NaOCl,is the active ingredient in household
Q157: Calculate the Ksp for silver sulfite if
Q158: What volume of 5.00 × 10-3 M
Q159: The equivalence point pH of the titration
Q160: What is the equilibrium constant expression for
Q162: What is the molar solubility of AgCl
Q163: The neutralization constant Kn for the neutralization
Q164: What is the least soluble salt of
Q165: Which set of ions precipitate as sulfides?
A)Ag+,Pb2+,Mn2+
B)Pb2+,Fe2+,Ca2+
C)Co2+,Ba2+,K+
D)NH4+,Na+,K+
Q166: The balanced net ionic equation for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents