Calculate ΔS° for the following reaction. N2(g) + 2 O2(g) → 2 NO2(g) 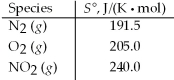
A) -156.5 J/K
B) -121.5 J/K
C) 15.5 J/K
D) 636.5 J/K
Correct Answer:
Verified
Q18: What is W in Boltzmann's formula,S =
Q19: The Boltzmann formula is S = k
Q20: Without doing any calculations,determine whether the standard
Q21: For the process CaCO3(calcite)→ CaCO3(aragonite)ΔH° = -0.21
Q22: Why is the sign of ΔG rather
Q24: The solubility of manganese(II)fluoride in water is
Q25: For bromine,ΔH°vap = 30.91 kJ/mol and ΔS°vap
Q26: ΔS° = -198.7 J/K for the reaction
Q27: Which statement is true about the formation
Q28: Calculate ΔS° for the formation of three
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents