The following pictures represent three equilibrium mixtures for the interconversion of A,B,and C molecules (unshaded spheres) into X,Y,and Z molecules (shaded spheres) ,respectively.What is the sign of ΔG ° for each of the three reactions? 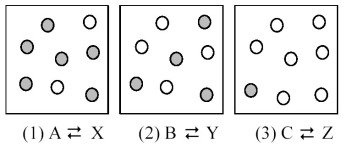
A) ΔG °(1) = -;ΔG °(2) = +;ΔG °(3) = 0
B) ΔG °(1) = -;ΔG °(2) = 0;ΔG °(3) = +
C) ΔG °(1) = 0;ΔG °(2) = -;ΔG °(3) = +
D) ΔG °(1) = +;ΔG °(2) = 0;ΔG °(3) = -
Correct Answer:
Verified
Q76: What is the relationship between ΔG,Qp,and Kp
Q77: In figure (1)below argon atoms,represented by unshaded
Q78: Q79: When equilibrium is reached at constant temperature Q80: In figure (1)below oxygen molecules,represented by unshaded Q82: The entropy change associated with the expansion Q83: Consider the following gas-phase reaction of A2 Q84: Which of the following processes is spontaneous? Q85: What is the entropy change associated with Q86: For which of the following will the![]()
A)a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents