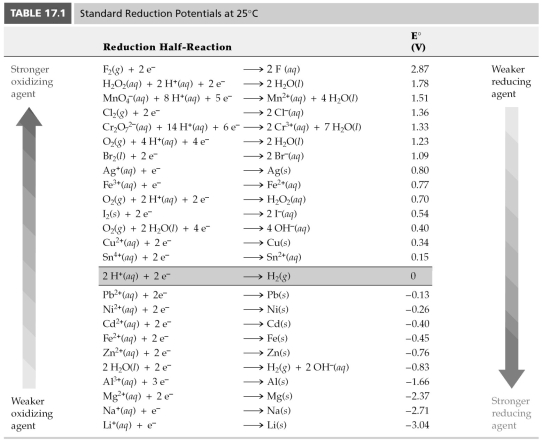
-According to Table 17.1,which aqueous metal ion will reduce Ag+,but not Cu2+?
A) Fe2+
B) Fe3+
C) Mn2+
D) Sn2+
Correct Answer:
Verified
Q55: Q56: Consider the following standard reduction potentials, Al3+(aq)+ Q57: Calculate the cell potential at 25°C for Q58: Consider the following table of standard reduction Q59: A galvanic cell consists of one half-cell Q61: Which statement below is not true? Q62: A cell based on the reaction below Q63: The cell reaction for a lead storage Q64: Which of the following reactions is most Q65: Ag+(aq)+ e- → Ag(s)E° = +0.800 V![]()
A)The cell
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents