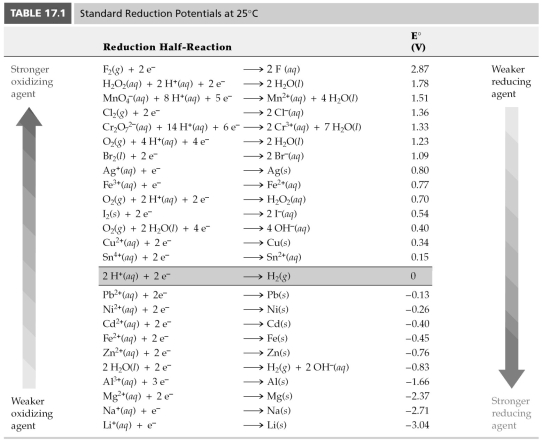
-Using Table 17.1,find E° for 2 H2O(l) → 2 H2(g) + O2(g) .
A) -2.06 V
B) -1.23 V
C) -0.80 V
D) -0.40 V
Correct Answer:
Verified
Q37: For a galvanic cell that uses the
Q38: In a galvanic cell constructed from Pb(s)|
Q39: What is the relation between joules (J),volts
Q40: For the hypothetical reaction A + 2
Q41: Consider the galvanic cell,Pt(s)∣ H2(1 atm)|H+(1 M)∣∣
Q43: Calculate the cell potential E at 25°C
Q44: What is the Al3+:Ag+concentration ratio in the
Q45: Given: Ag+(aq)+ e- → Ag(s)E° = +0.799
Q46: Based on the half-reactions and their respective
Q47: Based on the half-reactions and their respective
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents