When suspected drunk drivers are tested with a Breathalyzer,the alcohol (ethanol) in the exhaled breath is oxidized to acetic acid with an acidic solution of potassium dichromate: 3 CH3CH2OH(aq) + 2 Cr2O7-2 (aq) + 16 H+(aq) 3CH3CO2H(aq) + 4Cr+3(aq) + 11H2O (l)
What is the value of E for the reaction when the concentrations of ethanol,acetic acid,Cr2O7-2,and Cr+3,are 1.0M and the pH is 4.00? (Eº for this cell is 1.30V and the temperature is 298K) .
Equations to solve this problem are below.
Ε = ε° - 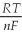 lnQ
lnQ
Ε = 1.30 - 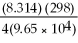 ln
ln 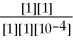
A) 1.62 V
B) 1.30 V
C) 0.98 V
D) 1.24 V
Correct Answer:
Verified
Q75: When a cell reaction reaches equilibrium,
A)E° =
Q76: For a particular cell based on the
Q77: The following cell has a potential of
Q78: Shown below are the reactions occurring in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents