Consider the following galvanic cell. 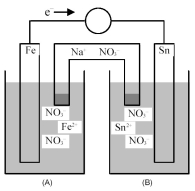
-Identify the anode and cathode,and indicate the direction of Na+ ion and NO3- ion flow from the salt bridge.
A) Fe is the anode and Sn is the cathode;Na+ ions flow into half-cell compartment (A) and NO3- ions flow into half-cell compartment (B) .
B) Fe is the anode and Sn is the cathode;NO3- ions flow into half-cell compartment (A) and Na+ ions flow into half-cell compartment (B) .
C) Sn is the anode and Fe is the cathode;Na+ ions flow into half-cell compartment (A) and NO3- ions flow into half-cell compartment (B) .
D) Sn is the anode and Fe is the cathode;NO3- ions flow into half-cell compartment (A) and Na+ ions flow into half-cell compartment (B) .
Correct Answer:
Verified
Q102: Q103: Shown below is a galvanic cell with Q104: Consider the galvanic cell shown below. Q105: Consider the following galvanic cell. Q106: Consider the galvanic cell shown below. Q108: Consider the following galvanic cell. Q109: Consider the galvanic cell shown below. Q110: Consider the following galvanic cell. Q111: NaNO3(aq)is employed in the salt bridge.Give the Q112: If the concentrations of Ag+(aq)and Cu2+(aq)are varied![]()



Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents