Consider the galvanic cell shown below. 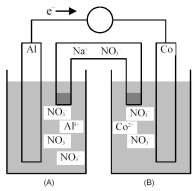
-What is the quantitative change in the cell voltage on increasing the ion concentration in the anode compartment by a factor of 10?
A) +0.03 V
B) +0.02 V
C) -0.02 V
D) -0.03 V
Correct Answer:
Verified
Q130: Shown below is an electrochemical cell with
Q131: What are the coefficients in front of
Q132: Q133: In the unbalanced equation shown below how Q134: What are the coefficients in front of Q136: What are the coefficients in front of Q137: Based on the balanced chemical equation shown Q138: What is the molarity of a potassium Q139: Consider the following galvanic cell. Q140: The initial concentrations of Ag+(aq)and Cu2+(aq)are both![]()

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents