Multiple Choice
Based on the balanced chemical equation shown below,determine the mass percent of Fe3+ in a 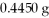 sample of iron ore,if 22.40 mL of a 0.1000 M stannous chloride,SnCl2(aq) ,solution is required to completely react with the Fe3+ present in the ore sample.The chemical equation for the reaction is: 2 Fe3+(aq) + Sn2+(aq) → 2 Fe2+(aq) + Sn4+(aq) .
sample of iron ore,if 22.40 mL of a 0.1000 M stannous chloride,SnCl2(aq) ,solution is required to completely react with the Fe3+ present in the ore sample.The chemical equation for the reaction is: 2 Fe3+(aq) + Sn2+(aq) → 2 Fe2+(aq) + Sn4+(aq) .
A) 8.982%
B) 14.06%
C) 28.11%
D) 56.22%
Correct Answer:
Verified
Related Questions
Q116: Fuel cells
A)produce carbon dioxide and hydrogen.
B)emit sulfur
Q117: Consider the galvanic cell shown below.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents
