Given that Br2(g) + 2 e- → 2 Br-(aq) is the reduction half-reaction for the overall reaction 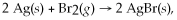 what is the oxidation half reaction?
what is the oxidation half reaction?
A) Ag(s) → Ag+(aq) + e-
B) Ag(s) + Br-(aq) → AgBr(s) + e-
C) Ag(s) + Br2(g) + e- → AgBr(s) + Br-(aq)
D) 2 Br-(aq) → Br2(g) + 2 e-
Correct Answer:
Verified
Q26: What is the shorthand notation that represents
Q145: What is the reduction half reaction for
Q146: Determine the number of water molecules necessary
Q147: A galvanic cell employs the reaction Ni2+(aq)+
Q148: In the relationship ΔG = -nFE°,what is
Q149: What is the reduction half-reaction for the
Q151: What is the balanced chemical equation for
Q152: In the shorthand notation for a galvanic
Q154: For the galvanic cell reaction,expressed below using
Q155: According to the balanced chemical equation 5
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents