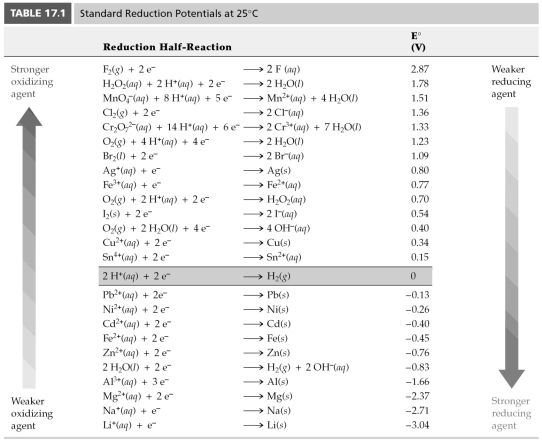
-Based on the following information, Cl2(g) + 2 e- → 2 Cl-(aq) E° = 1.36 V
Zn2+(aq) + 2 e- → 2 Zn(s) E° = -0.76 V
Which of the following chemical species is the strongest reducing agent?
A) Cl2(g)
B) Zn2+(aq)
C) Cl-(aq)
D) Zn(s)
Correct Answer:
Verified
Q25: What is the shorthand notation that represents
Q37: For a galvanic cell that uses the
Q156: What is the balanced equation for the
Q159: What is the balanced equation for the
Q160: For the galvanic cell reaction,expressed below using
Q162: Based on the following information, F2(g)+ 2
Q163: A particular 12V battery is based on
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents