The mass change for the reaction shown below is ________ amu.The atomic masses are 3He (3.0160),4He (4.0026),1H (1.0078).
2 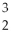 He →
He → 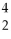 He + 2
He + 2 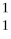 H
H
Correct Answer:
Verified
Q164: The mass defect for the chemical reaction
Q165: Among the types of radiation,α,β,γ,and X,the one
Q166: 201Tl is used in myocardial perfusion imaging.It
Q167: Radiocarbon dating involves the unbalanced equation shown
Q168: Carbon-14 has a half-life of 5715 years.Currently
Q170: What is needed to balance the nuclear
Q171: The two reactions shown below are classified
Q172: What is needed to balance the nuclear
Q173: In the transmutation reaction that results in
Q174: An average person receives 40 mrem of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents