When butane, 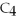
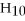 ,reacts with oxygen to produce carbon dioxide and water,2880 kJ of heat are released per mole of methane.How many kJ of heat are released per gram of oxygen used?
,reacts with oxygen to produce carbon dioxide and water,2880 kJ of heat are released per mole of methane.How many kJ of heat are released per gram of oxygen used?
A) 6.92 kJ/g
B) 13.8 kJ/g
C) 585 kJ/g
D) 1170 kJ/g
Correct Answer:
Verified
Q182: Identify the following amine base found in
Q183: Based on the structure of its side
Q184: Identify the following amine base found in
Q185: Identify the following dipeptide. Q186: Based on the structure of its side Q188: Based on the structure of its side Q189: How many isomers are there for C4H10? Q190: Identify the following dipeptide. Q191: Based on the structure of its side Q192: Based on the structure of its side![]()
A)2
B)3
C)4
D)5![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents