Consider the reaction shown and identify the statement that is not true.
CaCO3 (s) 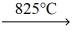
CaO (s) + CO2 (g)
A) This reaction is balanced as written.
B) The reactant must be heated for this reaction to occur.
C) The products are a solid and a gas.
D) Water must be present for this reaction to occur.
E) There are no solutions used in this reaction.
Correct Answer:
Verified
Q2: When the reaction shown is correctly balanced,the
Q4: Which is the correct equation for the
Q15: Which statement regarding balanced chemical equations is
Q16: When the reaction shown is correctly balanced,the
Q18: Which of the following equations is not
Q19: The balanced equation for the reaction occurring
Q24: Which reaction is not an example of
Q32: Which reaction is an example of a
Q35: The combination of ions least likely to
Q38: The combination of ions most likely to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents