Which is not a correct statement of Charles's Law?
A) For a gas sample at constant pressure,temperature and volume are inversely proportional.
B) volume α temperature
C) V/T = a constant
D) 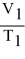
=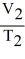
E) none of the above
Correct Answer:
Verified
Q2: Which combination of characteristics is most likely
Q14: Which transformation is freezing?
A)liquid → solid
B)liquid →
Q21: What would be the new pressure if
Q23: Which represents the largest pressure?
A)one atmosphere
B)five pounds
Q29: Which is not a correct statement of
Q34: Which assumption of Kinetic Molecular Theory is
Q36: Which measurement represents the smallest value of
Q47: A sealed container with gas at 2.00
Q55: A sample of gas has a volume
Q56: Sketch a graph illustrating Charles's Law.Use a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents