Hydrogen cyanide,HCN,is a weak acid.Which equation best represents its aqueous chemistry?
A) HCN (aq) + H2O (l) 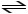
CN- (aq) + H3O+ (aq)
B) HCN (aq) + H2O (l) 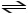
H2CN+ (aq) + OH- (aq)
C) HCN (aq) 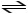
H+ (aq) + CN- (aq)
D) HCN (aq) 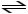
H- (aq) + CN+ (aq)
E) H2O (l) 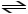
H+ (aq) + OH- (aq)
Correct Answer:
Verified
Q3: The classification of an acid or a
Q12: Acetic acid is a weak acid in
Q18: What is the conjugate acid of HSO4-?
A)SO42-
B)H2SO4
C)H3O+
D)OH-
E)H2SO3
Q22: What is the conjugate acid of water?
A)H2O(l)
B)H3O+(aq)
C)OH-(aq)
D)H+(aq)
E)O2-(aq)
Q24: Which compound has a value of Ka
Q28: At 25°C,the value of Kw is
A)1.00.
B)1.00 ×
Q30: Which one of the following is the
Q31: Which one of the following is the
Q35: Ammonia reacts with acids because
A)it contains the
Q40: Water and HSO4- can either accept protons
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents