The molecule shown is a ________ alcohol because ________. 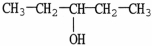
A) primary; it has one -OH group
B) primary; its -OH group is on the end of the molecule
C) secondary; the carbon bonded to the -OH group is bonded to two other carbons
D) secondary; each group bonded to the hydroxyl carbon contains two carbon atoms
E) tertiary; the -OH is bonded to the number 3 carbon
Correct Answer:
Verified
Q21: All of the following properties of alcohols
Q22: Compounds of the type RCH2-OH are referred
Q24: Which molecule shown is a tertiary alcohol?
A)
Q25: Which compound would have the highest boiling
Q28: The name of the alcohol shown is
Q30: Compounds of the type R3C-OH are referred
Q32: Which molecule shown is a secondary alcohol?
A)
Q33: Which compound has the lowest boiling point?
A)CH3CH2CH2CH2CH2OH
B)CH3CH2CH2CH2CH3
C)CH3CH2CH2CH2OH
D)CH3CH2CH2CH3
E)CH3CH2CH2OH
Q35: Which compound is a tertiary alcohol?
A)1-propanol
B)2-methyl-1-hexanol
C)2-methyl-2-hexanol
D)3-methyl-2-hexanol
E)3-hexanol
Q56: Which of the following would at best
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents