Multiple Choice
Which equation correctly represents the dissociation of a carboxylic acid in water?
A) CH3COOH + H2O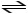
CH3CHCOOH2+ + OH-
B) CH3COOH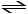
CH3COO- + H+
C) CH3COOH + H2O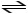
CH3COO- + H3O+
D) CH3COOH + H3O+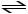
CH3COOH2+ + H2O
E) CH3COOH + 2 H2O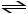
CH3COO2- + 2 H3O+
Correct Answer:
Verified
Related Questions
Q24: The solubility of compounds containing the carboxylic
Q26: An acyl group is a group in
Q27: Which of the compounds shown can form
Q28: What is the common name of the
Q29: What is the IUPAC name of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents